

Russia has decided to build its own factories for hemodialysis equipment. This comes after the largest U.S. manufacturer ceased shipments to the country. For years, Russian doctors have relied on Western equipment, and now they must adapt to substitutes. This is just one of the problems facing Russian medicine as a result of the country’s full-scale invasion of Ukraine. Several major pharmaceutical companies have pulled out of Russia, and the end of international clinical trials poses even more severe risks. Currently, 80% of Russian doctors are dealing with drug shortages and poor-quality medical supplies.
Content
No extra competitors
Russian insulin
Medical equipment: made in China
No international research
Hemodialysis, a treatment that filters the blood of patients whose kidneys are not working properly, is one of the most complex medical procedures there is. In April 2024, the U.S. pharmaceutical company Baxter, the world’s largest supplier of nephrology equipment, suspended its business in Russia, leaving doctors to get by with unfamiliar — and sometimes obsolete — technology. Soon, they will have to adapt to using Indian devices, which are set to be assembled at two factories in the Kaluga region. Construction of the facilities is scheduled to begin in September of this year.
“All this time, we worked with U.S. or German equipment that we knew well. Hemodialysis requires not only well-trained staff but also equipment that has been tested over the years. Almost no one currently has experience with Indian equipment,” said a Russian nephrologist in conversation with The Insider (the doctor requested anonymity for safety reasons).
No extra competitors
Back in 2015, after Western sanctions were imposed following Russia’s occupation of Crimea and its sponsorship of a hot war in the eastern Ukrainian Donbas region, Russian authorities aimed to substitute imported medical technologies with domestically produced ones. Initially, the plan was to localize the production of medicines and equipment as much as possible. This led to the “third wheel” principle: if two or more Russian manufacturers participate in state procurement, foreign companies are not allowed to bid. In 2021, this rule was tightened even further: now, if a manufacturer from another Eurasian Economic Union (EAEU) country participates in the tender, they automatically win over foreign competitors.
Russian authorities aimed to substitute imported medical technologies with domestically produced ones back in 2015, after the first Western sanctions were imposed
This approach drew serious criticism from Russia’s professional medical community. In February 2020, 160 oncologists and hematologists wrote a collective letter highlighting the side effects of domestic generics replacing original Western drugs. “A horrible thing is that the responsibility lies with the doctor, but the procurement is not handled by the doctor. According to Federal Law No. 44 [on procurement], the cheapest option must be purchased, not necessarily the best quality,” explains surgeon Alexander Vanyukov, the former head of a department at a Moscow city hospital.
Even at that time, there was worry that patients with specific intolerances would soon be unable to receive therapy due to the withdrawal of alternative drugs from the market.
Patients with specific intolerances would be unable to receive therapy due to the withdrawal of all alternative drugs from the market
Then, in the immediate aftermath of the onset of the full-scale war in February 2022, Western pharmaceutical companies began withdrawing from the Russian market one after another. According to a study by the professional community “Doctors.RF,” nearly 80% of Russian doctors have faced drug shortages.
The most common shortages are of antibiotics, insulins, antidepressants, neuroleptics, antiepileptics, and anticonvulsant drugs. In total, there have been reports of shortages of over 400 items, 74 of which are on the list of vital and essential medicines.
More than half of the surveyed doctors reported problems with medical equipment and consumables. Two-thirds of the respondents said that sanctions have affected their work, leading to drug shortages, issues with medical equipment and consumables, and general difficulties in the exchange of scientific knowledge.
Russian insulin
Diabetics were among the first to experience drug shortages. In early June 2024, the Swiss company Swixx BioPharma announced it would stop supplying Humalog insulin to Russia. Tens of thousands of people relied on it daily, with the most vulnerable being type 1 diabetics, most of whom are children. For them, insulin therapy is essential, as they do not produce their own insulin.
Parents are generally hesitant to switch their children to other insulins once compensation has been established. Furthermore, Humalog has a long-standing reputation for reliability, having been approved by the FDA back in 1996.
The Russian manufacturer Geropharm is set to replace Humalog with the biosimilar RinLiz. Geopharm is owned by the family of Peter Rodionov, a former Gazprom executive and Russia’s ex-Minister of Fuel and Energy. The company is gradually becoming a key producer of diabetes drugs in Russia. In 2023, it became the main supplier of insulin in the government sector.
Patients are concerned. Maria, a Moscow mother of a child with type 1 diabetes, shares her worries with The Insider: “The transition to Geropharm biosimilars is frightening. There are many negative reviews in the community. Some people have seen some success with biosimilars, but imagine being a parent who has to test an alternative on their own child. What if it doesn't work? What then?”
Globally, biosimilars are used in order to lower costs after a drug's patent expires. However, the main concern is their quality. Unlike generics — drugs with an active ingredient identical to the patented original — biosimilars are not exact replicas. While generics are chemically identical to their originals, biosimilars cannot achieve the same level of similarity due to the complexities of biotechnological products.
Patients active in diabetes support forums are already expressing concerns about side effects:
“I visited the endocrinologist yesterday to report an adverse reaction to RinLiz. I've been experiencing red spots on my body and ineffective insulin — it's like injecting water with no effect.”
“Good afternoon. Can someone advise how to handle the situation where we're being prescribed RinLiz, but my daughter has an intolerance? She immediately develops lumps and inflammation.”
“Rinfast is terrible. My blood sugar fluctuates unpredictably. I've used Actrapid, Novorapid, and now FiAsp. With the same dose, food, and activity, my blood sugar was stable (within a range of no more than 1 unit) with those after 2 hours. But with Rinfast, it either spikes, causes hypoglycemia, or is normal.”
There is no data available on comprehensive clinical trials for the Russian drug. According to Geropharm, the biological similarity between RinLiz and Humalog was demonstrated, but the trial involved only 28 healthy adult volunteers — i.e. people without diabetes.
Furthermore, there is no information about whether any patients with type 1 diabetes or any children participated in the studies, which aim to confirm that the antibody levels produced by a biosimilar are comparable to those produced by the original insulin. As such, the drug’s immunogenicity (its potential to provoke an immune response) remains unclear.
The Insider submitted an official inquiry to Geropharm but did not receive a response.
Some patients have begun importing the withdrawn Humalog from Germany through “gray” market channels, paying 15,000 rubles ($170) for a pack of five insulin pens. For comparison, before the company exited the Russian market, such a package cost only 1,800 rubles in a typical Russian pharmacy.
There are also supply issues with other insulins. In March 2024, ultra-fast insulin FiAsp from Danish company Novo Nordisk disappeared from pharmacies and state procurement lists. According to Kommersant, state procurements of this drug fell by 95% in the first quarter of 2024.
Meanwhile, Geropharm is rapidly expanding its share in a market that was previously dominated by foreign companies. The Russian firm has secured a compulsory license to produce analogs of Novo Nordisk's Ozempic, a drug protected by a patent in Russia until 2035. This license allows Geropharm to manufacture the drug without Novo Nordisk's permission.
Geropharm is also targeting diabetes medications from the French manufacturer Sanofi. The Russian company has already filed a lawsuit to obtain the license for Toujeo (insulin glargine, a long-acting insulin that provides basal glycemic control for 24 hours with a single injection).
Experts note that the Russian pharmaceutical market is currently 90% dependent on foreign pharmaceutical substances, and rare and expensive drugs can still be imported in limited quantities despite sanctions. “Since the drugs are still needed, especially for the 'elite' children, they will most likely be imported by the likes of the Circle of Kindness or the black market,” Alexander Vanyukov, the Moscow surgeon, suggests.
Medical equipment: made in China
Like other sectors of the Russian economy, healthcare is increasingly dominated by Chinese manufacturers. Not only pharmaceutical substances, but also medical electronics, are now being sourced from China. However, there are concerns among specialists and patients about the quality of these products.
Healthcare is increasingly dominated by Chinese manufacturers
Chinese companies have become major suppliers of continuous glucose monitoring (CGM) devices in many Russian regions. CGMs are crucial for monitoring blood sugar levels and preventing hypoglycemic comas in patients with type 1 diabetes.
Previously, this market segment was largely occupied by the FreeStyle Libre, made by the U.S. company Abbott. According to the analytical firm Headway, Abbott held an 89% share of the Russian market in 2022–2023. Then Chinese continuous glucose monitors (CGMs), such as Dr. Brinner, began to replace American models. However, quality concerns have sparked numerous complaints from patients. Here is a typical review of these products on social media:
“Dr. Brinner RGMS-II monitoring is not monitoring; it's a torture device! Three iron nails in the child's hand cause immense discomfort and pain; these nails are not flexible!!!! People with diabetes heal wounds more slowly than healthy individuals. Instead of one puncture, which already takes a long time to heal, there will now be three.”
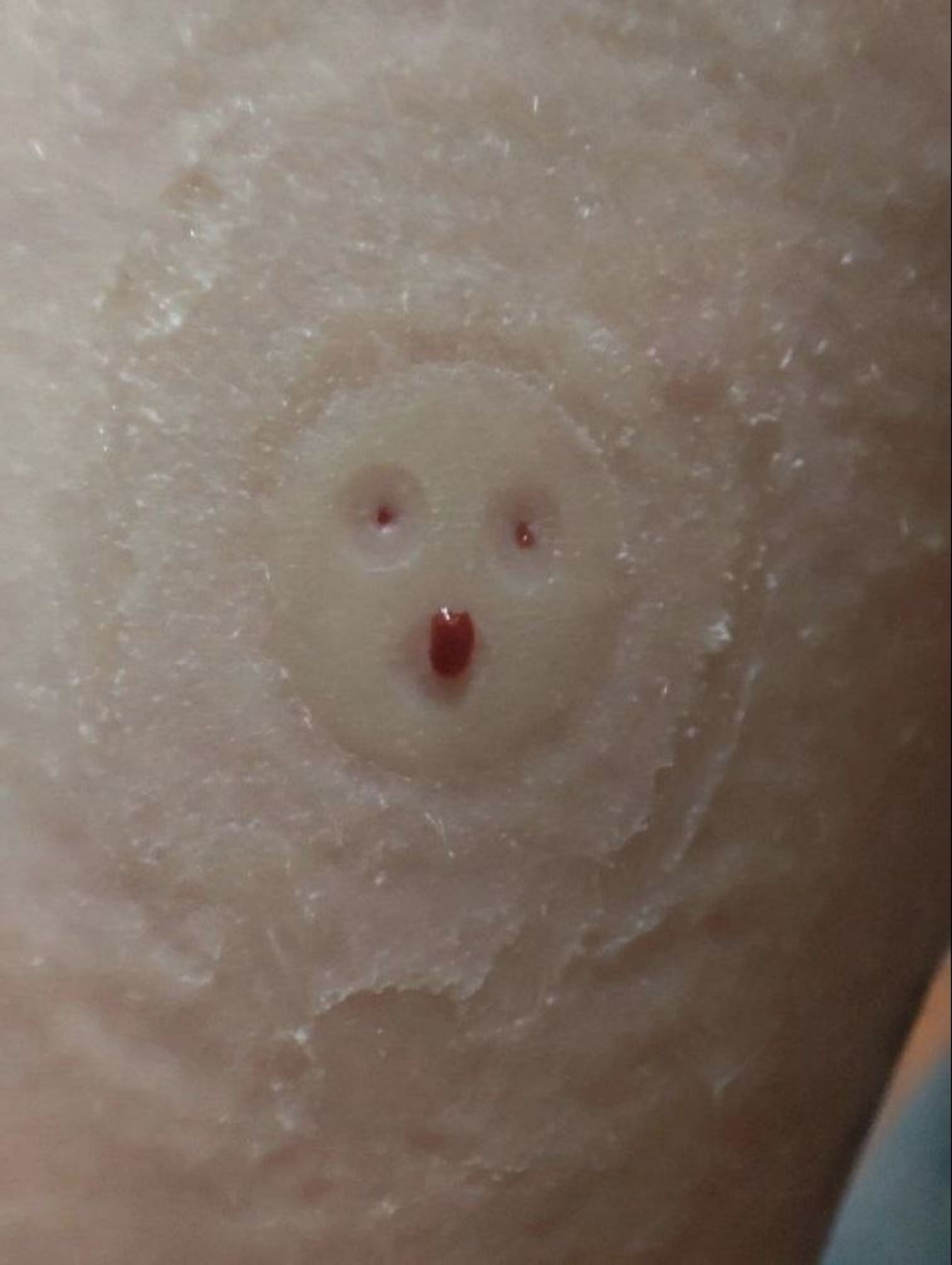
A photo of a child’s arm right after the removal the new Chinese continuous glucose monitor (Dr. Brinner) which shows the impact of the “three iron nails.”
In some regions, such as St. Petersburg and Novosibirsk, patients are filing complaints with health departments in large numbers or are refusing to use these medical devices altogether.
Doctors share the concerns of these frightened patients. “I don’t even want to use it myself, because I’ve seen inflammation from these three punctures. I feel sorry for my arm,” says an endocrinologist from a Moscow clinic in a conversation with The Insider.
In addition to inflammatory reactions, Chinese sensors face significant issues with accuracy. For people with type 1 diabetes, this is a critical issue, as insulin doses are based on the data provided, and precise calculation is essential. “I reviewed these Chinese and Korean monitors, and some of them don’t even list the MARD index [a measure of the accuracy of glucose monitoring],” says the doctor.
Resuscitation specialists have also encountered problems with Chinese products. The use of such supplies adds extra risk for patients, according to an anesthesiologist-resuscitator from Astrakhan (who asked to remain anonymous for safety reasons):
“Catheters for peripheral vessels have broken several times, leaving the end of the catheter in the patient's vein. We had to retrieve the remnants with the help of vascular surgeons. We also work with implantable intravenous port systems, and the Chinese set is practically impossible to install. It’s like a toy; it bends and falls apart in your hands.”
The quality of disposable supplies impacts the performance of ventilators: “Chinese contours did not fit the diameter; they were larger, resulting in a lack of airtightness.”
In addition to disposables, anesthesiologists have also started using Chinese drugs for anesthesia induction. “The propofol we’re used to, made from Chinese raw materials, requires double the dosage. Otherwise, it has no effect,” says a doctor.
However, not all Chinese products are the same, says surgeon Alexander Vanyukov: “There is high-quality Chinese equipment, but you don’t have the option to choose because the cheapest products are purchased. This issue isn’t even about the war or sanctions — it’s that the state procurement system was initially set up incorrectly.”
No international research
Previously, foreign companies conducted the second and third phases of clinical trials in Russia, which assessed the most effective drug dosages and administration methods. These trials tested cutting-edge medicines for treating severe diseases. Such research is crucial for introducing new drugs to the market. For many patients, participating in these trials was the only way to access innovative treatments.
The suspension of international clinical trials could have significant long-term effects on Russian medicine. Data from the Association of Clinical Research Organizations (ACRO), which includes major global pharmaceutical companies like Novo Nordisk, Pfizer, Bayer, and Novartis, shows that the number of clinical trials in Russia has dropped by 95% over the past three years.
Clinical trials are crucial for introducing new drugs to the Russian market
According to the association's report, only 18 studies were conducted in 2023, compared to 367 in 2021 — the year before the start of the full-scale war. However, there has been an increase in trials for generics and biosimilars. The main countries whose companies continue to conduct research in Russia are India (44%) and Belarus (26%).
As ACRO admits, “We have watched in real-time as everything that was so painstakingly built in Russia over the previous years — starting from the late 1990s — has collapsed. This was an industry of high-standard international clinical research. Two years ago, all hopes for further development of international multicenter clinical trials in the country vanished overnight.”
Additionally, on July 5, the Russian Ministry of Health canceled domestic Good Clinical Practice (GCP) regulations, with the order set to take effect on September 1, 2024. GCP is an international ethical and scientific standard for designing and conducting research. It ensures that the rights, safety, and well-being of research participants are protected in line with the principles of the World Medical Association’s Declaration of Helsinki, and it safeguards the reliability of clinical research data.
However, Vanyukov believes that, at this point, the cancellation of GCP regulations will have minimal impact: “They can't make it worse. International trials are halted, but [Russian interests] will be able to conduct their own trials — on military personnel or prisoners, for example.”